Mass spectrometry is a powerful analytical technique that can be used to identify and quantify the components of a sample. It is a versatile technique that can be used for a wide variety of applications. It is used in many different fields, including chemical analysis, drug discovery, environmental monitoring, and forensics.
There are several different stages involved in this process, and the stages can vary depending on the specific type of mass spectrometer used, and the information desired. Different mass spectrometry techniques, such as tandem mass spectrometry (MS/MS) or high-resolution mass spectrometry, may involve additional stages or modifications to the basic workflow. However, the overall principles of ionization, separation, analysis, detection, and data analysis remain fundamental to all mass spectrometry analyses.
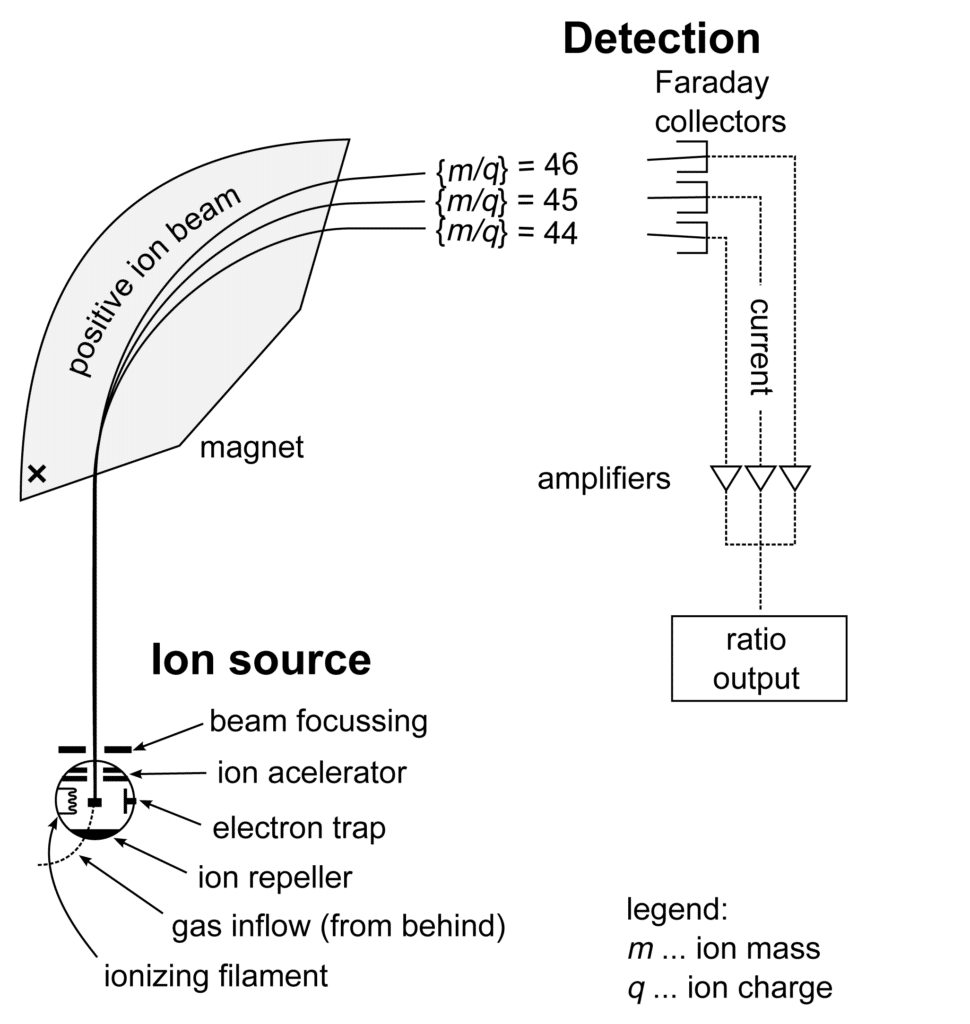
Mass Spectrometer Schematic
The Different Stages of Mass Spectrometry
1. Ionization
The first stage of mass spectrometry is to ionize the molecules in the sample. This is done by bombarding the molecules with electrons, photons, or other high-energy particles. The ionization process results in the formation of charged particles, or ions.
The ionization process can be destructive or non-destructive. Destructive ionization techniques completely break down the sample molecules into ions, while non-destructive ionization techniques only ionize a small fraction of the sample molecules.
There are many different ionization techniques that can be used in mass spectrometry. The most common ionization techniques include:
- Electron ionization (EI)
- Chemical ionization (CI)
- Matrix-assisted laser desorption/ionization (MALDI)
- Electrospray ionization (ESI)
Each method has its advantages and is suited for different types of samples. The choice of ionization technique depends on the type of sample being analyzed and the desired information.
2. Deflection/Ion Separation
Once the sample is ionized, the next stage is ion separation. This stage involves separating ions based on their mass-to-charge ratio (m/z). Several techniques are used for ion separation, including magnetic sector analyzers, quadrupole analyzers, time-of-flight (TOF) analyzers, ion traps, and hybrid instruments that combine different analyzers.
This is because ions of different masses and charges will be deflected differently by a magnetic or electric field.
For example, in a mass spectrometer, ions are first accelerated by an electric field into a bean of charged particles. The acceleration voltage is typically several thousand volts, and it causes the ions to acquire a high kinetic energy. The ion beam then passes through a magnetic field, which deflects them according to their mass (m/z ratio). The lighter the ions, the more they are deflected, and the heavier the ions, the less they are deflected.
3. Detection
The ions that have been deflected by the magnetic field are then detected by a detector. The detector converts the ions into an electrical signal, which is then amplified, analyzed, and recorded. The detector measures the m/z ratio of each ion. The m/z ratio is a unique identifier for each molecule.
The type of detector used in mass spectrometry depends on the application. Some common detectors include electron multipliers, ion counters, and quadrupole mass filters, among others. The choice depends on the type of ions and the mass analyzer used.
4. Data Analysis
Once the ions are detected and converted into electrical signals, the final stage involves data analysis. The recorded data is processed using specialized software. The resulting data is called a mass spectrum, which is a plot of the ion abundance (y-axis) versus the m/z ratio (x-axis). The mass spectrum provides valuable information about the composition and structure of the analyzed sample.
In modern mass spectrometers, these steps are managed by computer software.
Mass spectrometry is a versatile technique that can be used to answer a wide variety of questions about the composition of a sample. It is a powerful tool for scientists and researchers in a variety of fields.
The different stages of mass spectrometry are all essential for the accurate analysis of a sample. We hope this Tech Tip provides a good overview of the process. Learn more by following the links below.
Additional Resources:
- Introduction to Mass Spectrometer | Creative Proteomics
- What is Mass Spectrometry? | Broad Institute
- A Beginner’s Guide to Mass Spectrometry | ACD Labs
Shop our extensive inventory of high-quality, expertly refurbished mass spectrometers.